Abstract
Background: Analyses in myelodysplastic syndromes (MDS) and other acquired anemias suggest an association between iron overload and inferior clinical outcomes. There are minimal data examining iron levels in patients with acute myeloid leukemia (AML) and its relation to clinical outcomes. Patients with AML aged 60 years or older have inferior outcomes in general and no studies examine iron load and clinical outcomes in these patients. We wished to determine whether iron levels might contribute to the prognosis of these patients.
Methods: We performed a retrospective analysis of patients with AML aged ≥60 years diagnosed from 2002-2018. Patients were identified from the clinical database and charts reviewed. Clinical data extracted included baseline characteristics (demographics; AML presentation; ECOG performance status [PS]; comorbidities [Charlson Comorbidity Index (CI) and Hematopoietic Stem Cell Transplantation CI]; predisposing conditions; prior transfusions; blood counts; bone marrow findings; cytogenetic analysis) AML treatment received, status at last follow up and cause of death (COD). Serum ferritin level (SF) and bone marrow iron stores (BMIS) at AML diagnosis were recorded. BMIS were: absent, 0; reduced, 1; normal, 2; increased, 3; and markedly increased, 4. Elevated iron was: SF>750ng/mL or BMIS ≥3. Statistical analyses were performed using SPSS for Windows, version 25.
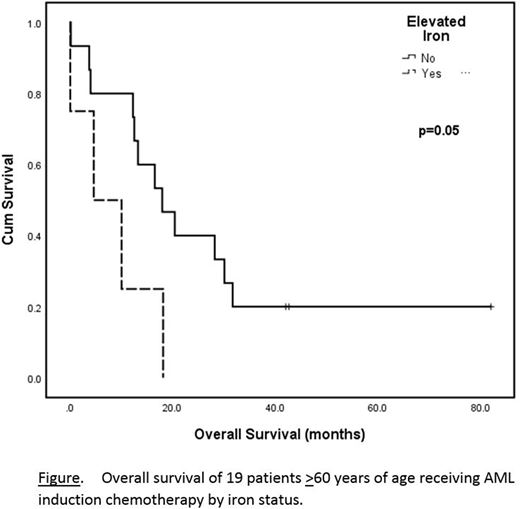
Results: Of 369 AML patients, 101 were ≥60 years and had adequate data for analysis. The median age at AML diagnosis was 70 (range 60-93) years and 60 (59.4%) were male. ECOG PS was 0, 1, 2, 3 and 4 in 7 (6.9%), 17 (16.8%), 48 (47.5%), 23 (22.8%) and 4 (4%) patients, respectively. 51 (50.5%) were de novo AML, and 49 (48.5%) had predisposing conditions, including MDS (n=39; 38.6%), MPN (n=7; 6.9%), and prior chemotherapy (CT) or radiation (n=3; 3.0%). Cytogenetic risk group was intermediate in 47 (46.5%) and adverse in 33 (32.7%). Treatment received included supportive care in 41 (40.6%), low dose chemotherapy (hydroxyurea, n=18; low dose cytarabine, n=4) in 22 (21.8%), induction CT in 18 (17.8%), and azacitidine in 20 (19.8%). Infection or inflammation were documented in 8 (7.9%). Comorbidities were present in 43 (42.6%) patients, and the median (range) number of comorbidities per patient was 1 (1-3). SF was available in 55 (54.5%), BMIS in 68 (67.3%), and both in 22 (21.8%). There was a significant correlation between SF >750ng/mL and BMIS ≥3 (r=0.555, p=0.008). A composite score including SF and BMIS revealed elevated iron stores (ELFE) in 39 (38.6%) and normal to decreased iron stores in 62 (61.4%). In univariate analysis, factors significant for overall survival (OS) included ECOG PS, p<0.0001; de novo versus secondary AML, p=0.02; neutrophil count, p<0.0001; platelet count, p<0.0001; bone marrow blast %, p=0.04; cytogenetic risk group, p=0.02; and AML treatment received, p<0.0001. Factors with a trend toward significance included the presence of dysplasia, p=0.07; and ELFE, p=0.07. In pairwise comparisons, dysplasia and AML treatment received lost significance, p=0.09. Factors remaining significant for OS when paired with ELFE included ECOG PS, p=0.02; and AML treatment (trend), p=0.05. In a multivariate analysis including ELFE, ECOG PS and AML treatment, ELFE and ECOG PS were significant for OS: p=0.046, hazard ratio (HR) for death 2.5, 95% confidence intervals (CI) 1.02-6.14, and p=0.006, HR=4.1, 95% CI=1.50-11.36, respectively. The median OS for patients receiving induction CT with no ELFE was 18 (range 0.2-82) months versus 10.1 (0.1-18.2) months with ELFE (p=0.05, see Figure). Baseline characteristics in the subgroup receiving induction CT were not significantly different between ELFE non-ELFE patients, with the exception of hemoglobin (p=0.008). COD in all patients were: AML progression, n=72 (71.3%); infection, n=4 (4.0%); and bleeding, n=4 (4.0%); with no significant difference in COD between patients with and without ELFE (p=0.4).
Conclusions: In this retrospective, non-controlled analysis, for patients aged ≥60 years with a new diagnosis of AML, elevated iron appears to be associated with inferior overall survival, particularly in those receiving induction chemotherapy. To our knowledge, these are the first data examining clinical outcomes in AML patients ≥60 years of age according to iron status. These results should be confirmed in larger, prospective analyses.
Leitch:AbbVie: Research Funding; Alexion: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal